Are you ready to find 'how to write net ionic equations for double replacement'? You will find all of the details here.
Ultimate ionic equation Atomic number 47 + (aq) + Cl-(aq) AgCl (s) – Complete symmetrical chemical formula equation-describes double replacement chemical reaction – Complete geographic region equation-• more accurately shows the reacting species as ions and the products either as ions or a hurried – Net geographical area equation-• focuses alone on the ions REACTING
Table of contents
- How to write net ionic equations for double replacement in 2021
- Single replacement reaction equations
- Net ionic equations examples
- Double replacement reactions net ionic equations worksheet
- Net ionic equation examples with answers
- Balanced net ionic equation
- Net ionic equation calculator
- Net ionic equation quiz
How to write net ionic equations for double replacement in 2021
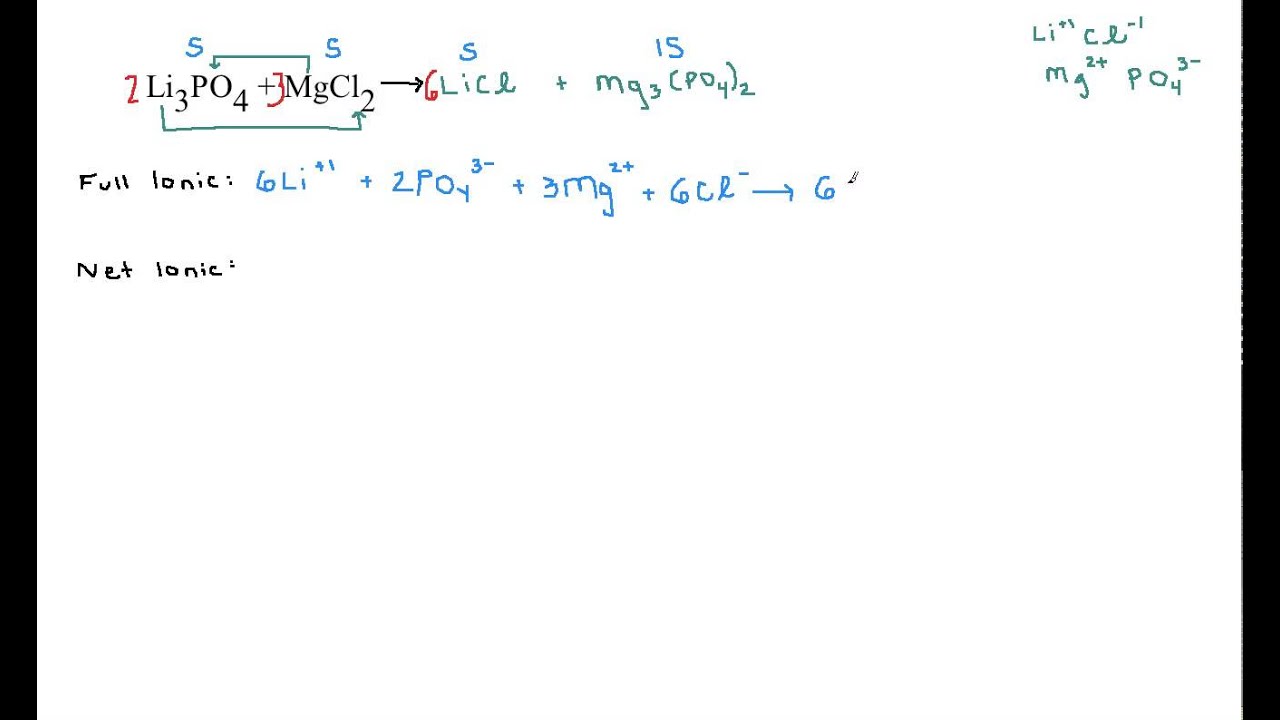 This picture representes how to write net ionic equations for double replacement.
This picture representes how to write net ionic equations for double replacement.
Single replacement reaction equations
 This picture illustrates Single replacement reaction equations.
This picture illustrates Single replacement reaction equations.
Net ionic equations examples
 This picture shows Net ionic equations examples.
This picture shows Net ionic equations examples.
Double replacement reactions net ionic equations worksheet
 This image representes Double replacement reactions net ionic equations worksheet.
This image representes Double replacement reactions net ionic equations worksheet.
Net ionic equation examples with answers
 This image illustrates Net ionic equation examples with answers.
This image illustrates Net ionic equation examples with answers.
Balanced net ionic equation
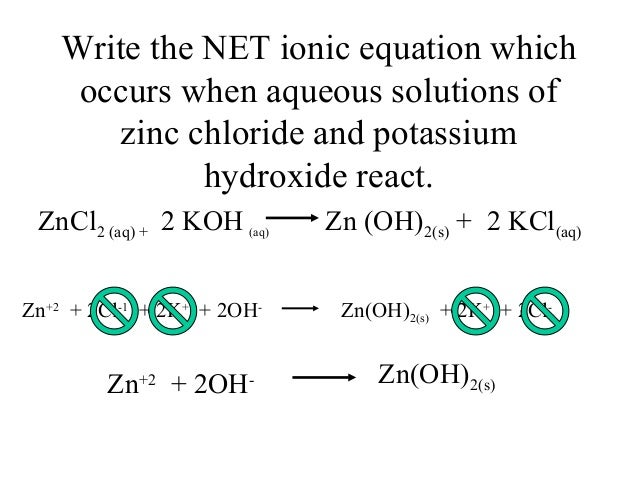 This image illustrates Balanced net ionic equation.
This image illustrates Balanced net ionic equation.
Net ionic equation calculator
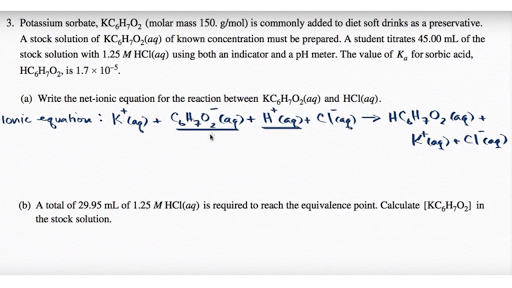 This picture representes Net ionic equation calculator.
This picture representes Net ionic equation calculator.
Net ionic equation quiz
 This picture shows Net ionic equation quiz.
This picture shows Net ionic equation quiz.
Which is the product of a double replacement reaction?
One product of a double replacement reaction (also called double displacement and metathesis) must be a solid (precipitate), an insoluble gas, or water. We can write net ionic equations for all of these. Example 1: Reaction between aqueous potassium chloride and aqueous lead (II) nitrate with one product as precipitate.
Is there a net ionic equation for a double replacement reaction?
We can write net ionic equations for all of these. However, if there is no precipitate, gas, or water formed, you would write NR for no reaction and there would be no net ionic equation. One product of a double replacement reaction (also called double displacement and metathesis) must be a solid (precipitate), an insoluble gas, or water.
Can a net ionic equation be written for no reaction?
However, if there is no precipitate, gas, or water formed, you would write NR for no reaction and there would be no net ionic equation. One product of a double replacement reaction (also called double displacement and metathesis) must be a solid (precipitate), an insoluble gas, or water. We can write net ionic equations for all of these.
Last Update: Oct 2021